The Compliant Software
for your Data Management needs
- Pharma
- Labs
- Biotechs
- Chemical
- Food & Beverage
- Cosmetics
Solutions
Quality
Manufacture
Clinical
Regulatory
Product Development
Applications
QMS
QMS-4-SME, a complete solution for Biotechs and startups in quality Management.
CAPA
Manage your deviations and CAPAs for continuous improvement.
Change Control
Collaborate and coordinate changes for increase efficiency.

Non-Conformance
Assure compliance and follow up with regulation requirements.
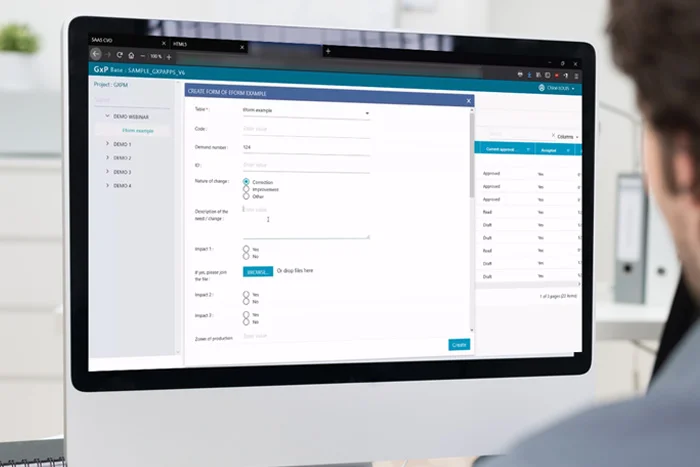
Forms Digitilization
GxpForms update your forms to unlimited possibilities.
Audit Management
Manager your internal and external audits easly and simply.
HSE Management
All your HSE activities are in one single point to collaborate and report.
Training Management
Records and centralize all activities related to your company's skills management.
Document Management
Structured Electronic Document Management system.
Excel Digitization
GxpXL allows you to protect your data while respecting compliance.
Validation Master Plan
Optimize and control your validation activities and planning.
Risks Analysis
Manage operational risk tracking, mitigation, evaluation, and monitoring.
Test & Validation
GxpSVV - Centralize and secure your testing, verification and validation activities.

Laboratories Management
GxpLab - Digitize your data entry sheets with guaranteed data integrity and traceability.
Analytics & Dashboard
GxpAnalytics - A powerfull dashboard customized with your KPIs.
GxpManager Platform's Native Functionalities
- Compliance
- Versioning
- Audit Trail
- Electronic Signatures
- Traceability Matrixes
- Automatic publications
- Workflows
- Dashboards
- 360° views
- Multi-lingual - French, English, German
The GxpManager platform is agile and complies with all business processes in industries with strong regulatory constraints.
Information is facilitated and secured to enable data management through powerful functions.
Complies with 21 CFR part 11 of the FDA, Annex 11 of the EU, ISO, GMP,…
SaaS Architecture (Cloud) with data hosted in a couple of trusted sovereign data centers based in France and certified HDS Tier 3 with ISO 27001 certification.
About Us
Software publisher since 2003, GxpManager offers a No Code Low Code solution in SaaS architecture for companies who need to process and manage critical data, such as in the life sciences, chemistry, food and beverage sectors…
Develop without programming
The goal is to develop applications without programming that meet all the requirements around critical data digitization in the company. Critical data can be inspected at any time by customers, regulatory bodies or normative authorities.
GxpManager est certifié ISO 9001:2015

Benefits
100% secured
Datacenters located in France.
HDS and ISO 27001 certified
Subscription
Various level of licences on annual subscription, renewable.
Rapid & Easy
Fast implementation with No Code Low Code deployment.
Compliant
Ensure regulatory compliance: FDA 21 CFR part 11, GMP appendix 11, and more.
Client Testimonials
“The use of GxpManager for our inventory management application brings us both full compliance with regulations and also flexibility and ease of use for users.
The rigorous work of the GxpManager development team and the quality of the work provided allowed us to properly understand the validation of our application..”

Applications Inventory of equipment to be qualified and Laboratory Transfer Management – SANOFI R&D – QA Department
“In order to continue its digital expansion on construction sites, Arkema’s Technical Department has chosen the GxpManager solution to centralize safety prevention data entries. The application, which has been custom-configured and renamed Gx-Prevention at Arkema, now provides a real-time view of safety events and supplier co-activity. For this project, the GxpManager teams demonstrated their seriousness and great responsiveness. ”

Arkema Technical Department – Service Engineering, Construction & Project Control (EC&PC)
“The digitalization of our quality processes is a major stake in the management and improvement of our field operations. The speed of implementation of an adapted solution with GxpManager was an essential point in our choice of partner. The application offers great customization opportunities and can be adapted to projects of any size. In addition, the commitment and availability of the GxpManager teams was a strong point that allowed us to achieve a custom project that met our expectations.”

Danone Nutricia – Marion MARGUERITTE – Quality Engineer