Solutions
Platform
Case studies
- Cell culture service digitizationThe application enables the planning of cell cultures and ensures the traceability of each associated action.
- Biological samples traceabilityImplementation of a custom stock management solution
- Artworks: Management of packaging items and promotionsManagement and validation of Artwork in-house up to the publication for the printer (subcontractor) for an international pharmaceutical company
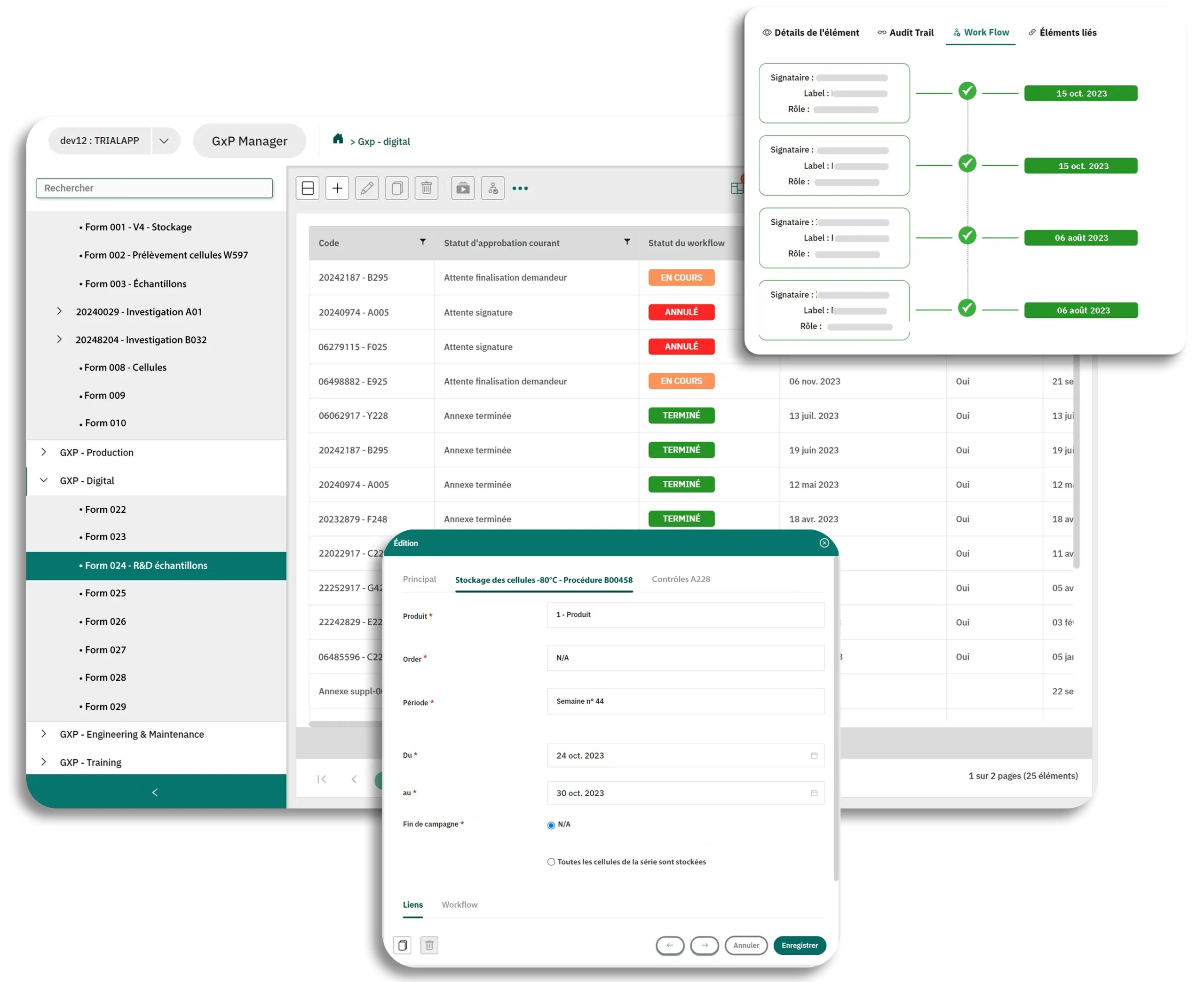
- Client Case Merck®: Digitalization of FormsDigitize more than 1000 paper forms without altering the content and signature processes...
- Quality Management System for a pharmaceutical logistics companyThe application enables simplified management of quality processes and regulatory compliance with 21 CFR part 11
- Inventory management of computerized systems equipmentCreate a regulatory obligations compliant application which simplifies the inventory process management.
- Management of the laboratory Equipment Moving ProcessIdentify all the steps required to move equipment and structure an associated project management approach.
- Master Data Management (MDM)Digitize the collection, verification and approval of Master Data.
- Discover our Use Cases
Discover how companies rely on GxpManager platform to meet their needs
All use cases
Resources
- News
- The 6 benefits of digitalizing audit managementDigitalization improves the efficiency and security of audits and enhances transparency and data traceability.
- Evolution of the Cosmetic Industry in the USA: Quality and ComplianceThe digitalization of companies in the cosmetics sector is becoming essential for increasing productivity and ensuring regulatory compliance.
- Modernization of Cosmetics Regulation Act in the US: Key ChangesMoCRA introduces essential changes in the American cosmetics industry
- Webinars on demand
- How to build and implement an eQMS: efficiency, compliance and data integrityHow to build and implement an eQMS to streamline your decision-making
- Digitization in Transport/logistics: Revolutionizing supply chain managementIn the transportation and logistics sector, adopting efficient tools to modernize and optimize their quality processes, is of critical importance
- GxpQMSThe Webinar presents the QMS-4-SME (Quality Management System for Small and Medium Enterprises)
- Customer testimonials
- Client Case Merck®: Digitalization of Forms
- Sanofi
- Athena IPS
- CRCDC Nouvelle-Aquitaine
- Resource center
Other news are available in our resource center
View all resources