Centralize, automate, and accelerate the management of your packaging materials and advertisements.
Eliminate inefficiencies in traditional processes by offering rigorous version control, automated approval workflows, integrated compliance tools, and improved team collaboration.
Centralized artwork management
Eliminate information silos and simplify data access for all stakeholders: everything is organized, secured, and accessible in one place. This centralization saves time while reducing errors associated with decentralized information management.
Version Control
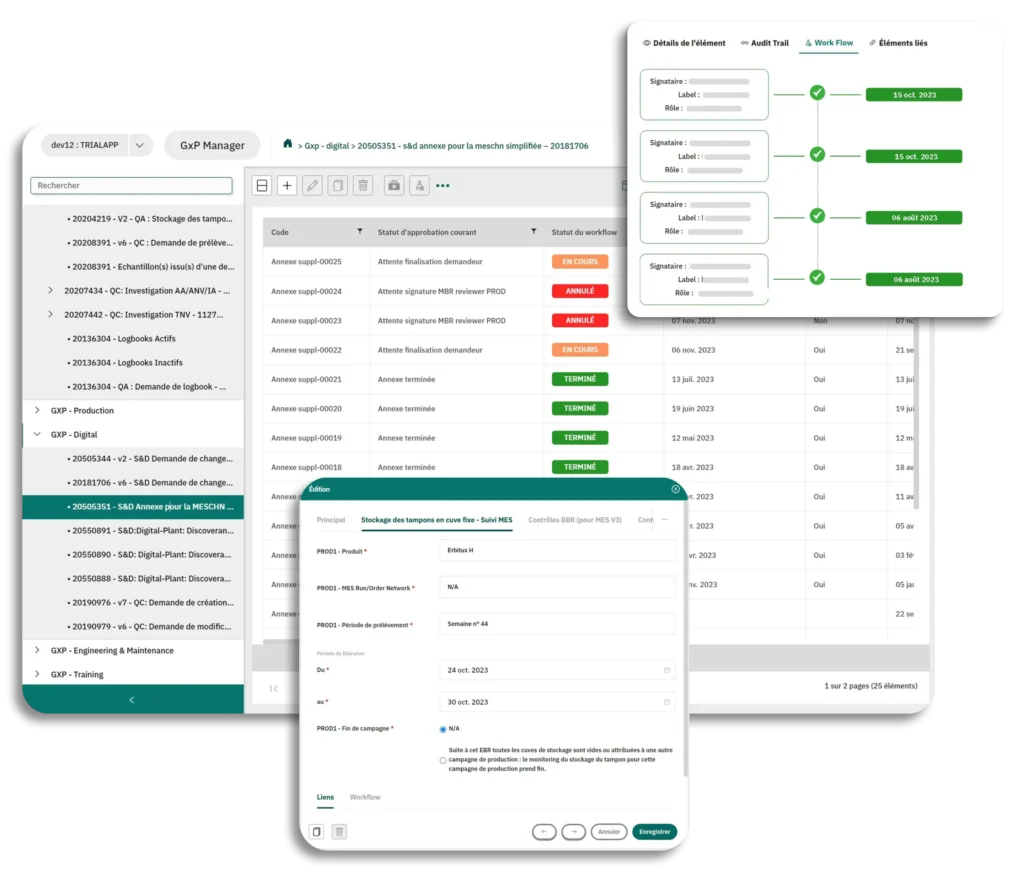
The version control system ensures that each team works with the latest, correct version of a document. It tracks and manages every modification made to artworks, with a detailed version history, ensuring optimal flexibility in document management.
Workflows & centralized approvals
Approval processes are centralized, simplifying the management of complex workflows. Each document goes through predefined approval steps. The process’s transparency allows all users to track the progress of approvals in real-time, thus reducing the risk of delays.
Simplified collaboration
By bringing all stakeholders together on a single platform, the application facilitates smooth and transparent communication, accelerates validation processes, and ensures that all participants are on the same objectives and guidelines.
Regulatory Compliance
Any regulated company’s priority is ensuring that every document complies with applicable standards and regulations. The application helps you identify potential issues before they become obstacles, avoiding non-compliance risks. It complies with the FDA’s CFR 21 Part 11, EU Annex 11, and national and international regulations, ISO standards.
Publishing and Document Distribution
The application facilitates the publication and distribution of final versions ready to be shared, whether internally or with external partners, such as printers or subcontractors.