Increasingly demanding certification processes
Regulatory compliance is essential in the field of medical devices to ensure the safety of patients as well as the effectiveness and quality of products. Manufacturers are responsible for demonstrating the compliance of their devices, a process that can be lengthy and demanding. Furthermore, the regulations governing the medical device sector (MDR) constantly evolve: regulation 2017/745, ISO 14971, IEC 62304, FDA QMR, 21 CRF part 11, CE marking, clinical evaluation…
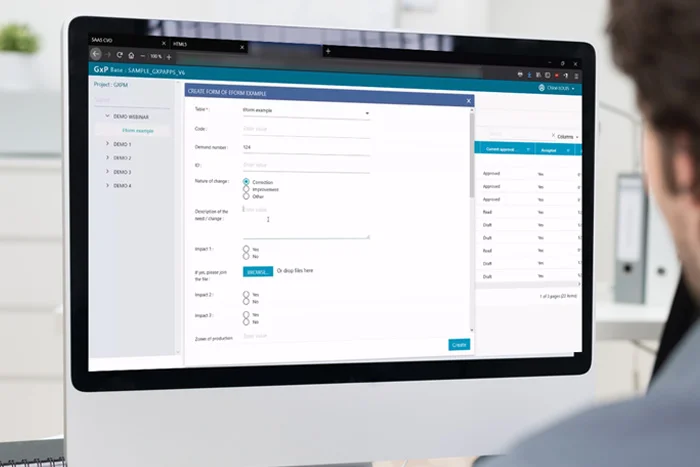
GxpManager application solutions help you demonstrate and ensure compliance throughout the product lifecycle, from design to manufacturing and beyond, with post-market surveillance.
Our solutions are designed to meet the needs of medical device manufacturers:
Our solutions meet the needs of medical device manufacturers
- Applications that are natively compliant and pre-qualified to meet the requirements of highly regulated sectors: Infrastructure qualification, IQ and OQ platform qualification (drastically reduces the cost of validating applications generated with GxpManager)
- Agile solutions capable of adapting to constant regulatory changes